Abstract
Introduction: The role of autologous stem cell transplantation (ASCT) as first-line therapy for newly diagnosed transplant eligible (NDTE) patients with multiple myeloma remains under evaluation. The primary objective of this study was to evaluate the progression-free survival (PFS) according to MRD status of patients achieving ≥VGPR following induction therapy followed by no further treatment. Those who achieved PR proceeded to ASCT. PADIMAC (ISRCTN no: 03381785) was designed to provide baseline data for a future response-adapted therapy trial in NDTE patients.
Methods: This single arm phase 2 trial was conducted at 13 UK sites. Patients eligible for ASCT received PAD (bortezomib (BZ), doxorubicin and dexamethasone) for 4-6 cycles. Those achieving <PR were off protocol. All others had PBSCH followed by restaging including MRD assessment by multi-parameter flow cytometry (MPFC) (10-4). Those in PR proceeded to ASCT (no maintenance, "PR-ASCT"), those achieving ≥VGPR stopped treatment ("VGPR-W&W"). Responses were assessed at 100 days post-PBSCH (including MRD), and monthly for up to 2 years. High risk disease was defined as ≥1 adverse FISH lesions [t(4;14), t(14;16), t(14;20), del(17p13), +1q21]. Initial results were presented at EHA 2017. Here we present final analysis including preliminary PFS2 for VGPR-W&W patients.
Results: From March 2011 to January 2014, 153 patients were enrolled (median age 55, range 28-71 years). The majority (135, 88%) received SC only BZ whilst 18 (12%) received at least 1 cycle IV, 139 (91%) received between 4-6 cycles of PAD. 89 (67%) patients were standard risk and 43 (33%) were adverse risk (21 missing). 91 (59%) reported ≥Grade 3 adverse event, mainly haematological: neutropenia 18%; thrombocytopenia 7%. Grade 1-2 neuropathy was similar for patients who received IV (55.6%) or SC (60%) BZ; however grade 3 neuropathy was lower with SC (7% vs 28% with IV).
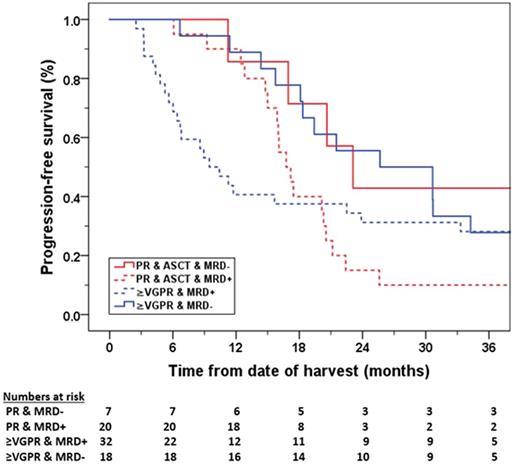
The overall response rate to PAD was 82%, 27 patients came off study prior to PBSCH (17 stable disease, 8 progressive disease (PD), 1 died, 1 withdrew). 19 patients came off study post-PBSCH (7 PD, 6 toxicities, 1 allograft, 5 withdrawal for other reasons). 63 (41%) patients achieved ≥VGPR post PBSCH, and 44 (29%) patients achieved PR of whom 36 proceeded to ASCT. After a median follow-up of 47.5m, median PFS for VGPR-W&W was 18.1m (95% CI: 12.7-23.6) ); and 15 (24%) patients remain alive and progression-free for >24m. Patients who were MRD neg at d100 (n=18) had a median PFS of 25.7m vs 9.5m for MRD pos (n=32), 13 missing. For PR-ASCT, the median PFS was 20.3m (95% CI: 19.7-20.9); 16.8m for MRD pos (n=20), whilst only 7 were MRD neg at day 100 (missing=5). Overall, MRD status at day 100 correlated with PFS (MRD neg: 25.7m vs MRD pos: 15.6m, p<0.05) and was independent of treatment (see Figure). Median % neoplastic plasma cells by MPFC post PBSCH were higher for PR-ASCT than VGPR-W&W patients: 0.36% (0.00 - 13.0) vs 0.02% (0.00-0.96), but similar at day 100 post PBSCH (0.04% (0.00 - 5.00) vs 0.01% (0.00 - 42.0). Patients with adverse risk cytogenetics had at least as deep responses as standard risk patients (≥VGPR in adverse risk: 69% vs standard risk: 54%), but PFS appeared shorter (16.0m versus 20.3m, p=0.25). This was also observed in the VGPR-W&W sub-group (14.4m vs 18.1m, p=0.25). Of the VGPR-W&W patients, 48 (76%) fulfilled IMWG criteria for progressive disease at time of analysis and 27 (56%) proceeded to ASCT at relapse. The PFS2 for VGPR-W&W was: 46.9m. Again, an association with MRD at day 100 was observed (p=0.01): 2-year PFS2 was 96.0% in MRD neg vs 80.7% (95% CI: 69.9-91.5) for MRD pos patients. The 2-year OS was 91.9% for VGPR-W&W (median OS not yet reached).
Conclusions: This is the first trial to investigate the outcomes of patients who achieve ≥VGPR following up to 6 cycles of induction therapy and receive no further treatment. Patients who were MRD pos at day 100 had a particularly poor outcome (median PFS of 9.5m) as did those with adverse cytogenetics (14.4 months), signifying the need for further treatment. However, those who were MRD neg at day 100 had a longer median PFS of 25.7m, which was similar to the median PFS of the PR-ASCT group. MRD status at day 100 post PBSCH was associated with PFS irrespective of treatment. These data support designing response adapted trials according to MRD status rather than serological response.
Popat: Celgene: Honoraria, Other: Travel support for meetings; Amgen: Honoraria; Takeda: Honoraria, Other: Travel support for meetings; Janssen: Honoraria, Other: Travel support for meetings. Cavenagh: Janssen: Honoraria; Celgene: Consultancy; Novartis: Consultancy; Takeda: Consultancy. Streetly: Janssen-Cilag Pharma: Speakers Bureau. D'Sa: Janssen Cilag: Consultancy, Honoraria, Other: Education grant, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Cook: Celgene: Honoraria, Other: Travel support, Research Funding; Janssen: Honoraria, Other: Travel support, Research Funding; Myeloma UK: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Other: Travel support; Takeda: Honoraria; Jazz Pharmaceuticals: Honoraria. Rabin: Celgene: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Takeda: Consultancy, Other: Travel support for meetings, Speakers Bureau; Janssen: Consultancy, Other: Travel support for meetings, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Owen: Takeda: Honoraria, Other: travel support; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Other: travel support. Yong: Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal